The World Health Organization (WHO) has published its first ever list of antibiotic-resistant bacteria that pose the greatest threat to human health and which urgently need new types of antibiotics. These bacteria are resistant to some last-resort antibiotics.
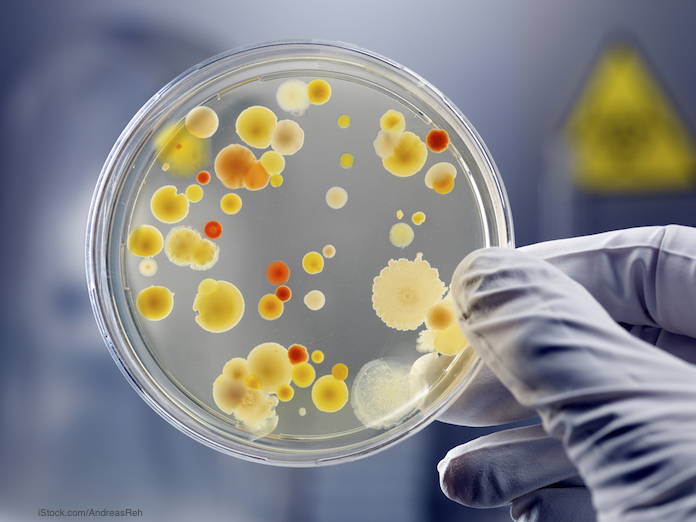
According to the Centers for Disease Control and Prevention (CDC), antibiotic-resistant bacteria kill 23,000 Americans every year. And antibiotic resistant bacteria are present in every country on earth.
The list highlights gram-negative bacteria that are resistant to multiple antibiotics. These pathogens are constantly evolving to resist new drugs, and they can pass genetic material to other bacteria so they can become drug resistant too.
Dr. Marie-Paule Kieny, WHO’s Assistant Director-General for Health Systems and Innovation said in a statement, “This list is a new tool to ensure R&D responds to urgent public health needs. Antibiotic resistance is growing, and we are fast running out of treatment options. If we leave it to market forces alone, the new antibiotics we most urgently need are not going to be developed in time.”
The list is divided into three categories according to the urgency of need to new drugs: Critical, high, and medium priority. The most critical group includes Enterobacteriaceae, which includes Klebsiella and E. coli. Those pathogens can cause severe and often deadly bloodstream infections. Those bacteria have developed resistance to third generation cephalosporins and carbapenems, which are the best available antibiotics for treating multi-drug resistant bacteria.
The second category tier includes Staphylococcus aureus, Helicobacter pylori, fluoroquinolone-resistant Campylobacter, and fluoroquinolone-resistant Salmonella. The third tier includes fluoroquinolone-resistant Shigella bacteria. All of those bacteria are familiar to those in the food safety industry.
WHO hopes that this list will spur governments to advance policies that incentive basic science and advanced R&D by publicly funded agencies such as the FDA and CDC, and by the private sector. Professor Evalina Tacconelli, head of the Division of Infectious Diseases at the University of Tübingen said, “new antibiotics targeting this priority list of pathogens will help to reduce deaths due to resistant infections around the world. Waiting any longer will cause further public health problems and dramatically impact patient care.”
WHO ended its news release by stating that while more research and development is vital, it can’t solve the problem alone. We must prevent infections by these pathogenic bacteria, and use antibiotics in both humans and animals appropriately. And when new antibiotics are developed, they must be used rationally and not overprescribed
Source=https://foodpoisoningbulletin.com/2017/who-publishes-list-of-bacteria-that-need-new-antibiotics/
FUMZYME , HERE TO FIGHT FUMONISIN IN LIVESTOCK
EFSA acknowledged the efficacy of the enzyme (FUMzyme®) in safely degrading fumonisins to non-toxic compounds in the gastrointestinal tract of poultry, as demonstrated in numerous feeding trials, stating that the product has the capacity to degrade fumonisins in feed, at concentrations below the Guidance limits operating in the EU in chickens and turkeys for fattening and laying hens at the minimum recommended dose of 15 U/kg complete feed.
Protecting flocks
Recent Biomin Mycotoxin Survey results of 3065 samples taken in the first half of 2016 indicate that fumonisins were detected in 80% of maize, 27% of wheat, 66% of finished feed and 40% of soybean meal samples. “We know that fumonisins impair the health and performance of poultry,” explains Ursula Hofstetter, Director Competence Center Mycotoxins at Biomin. Recent research revealed that the ingestion of fumonisins at levels below the EU recommended value (20 parts per million) can affect the expression of proteins related to pro- and anti-inflammatory responses in the intestinal tract of broilers. Levels of 20 ppm of fumonisins induce a higher excretion of Eimeria, the parasites responsible for coccidiosis. “In large-scale farms, this may promote parasite transmission between birds due to the high density of the animals,” she explained.
Biotransformation
The enzyme was originally isolated from the fumonisin degrading soil bacterium Sphingopyxis sp. MTA 144 and identified as fumonisin esterase by the Biomin Research Center. The purified-enzyme biotransforms fumonisins specifically and irreversibly into non-toxic metabolites. “Biotransformation is the most cutting-edge strategy to detoxify mycotoxins,” said Ms Hofstetter. Biotransformation works by transforming non-adsorbable mycotoxins into harmless substances without any side effects for livestock. “It’s the future of mycotoxin risk management,” she added.
Biomarkers
Demonstrating the effectiveness of the enzyme involved evidence using the serum sphinganine/sphingosine ratio, a key biomarker that indicates fumonisin exposure in animals. “This ratio was significantly reduced by the addition of the enzyme at the minimum proposed dose when added to diets contaminated with fumonisins,” according to the EFSA opinion.
Source=http://www.allaboutfeed.net/Mycotoxins/Articles/2016/11/Positive-EFSA-opinion-for-Fumzyme-2921861W/
Sept 2017
MOSANTO HAS JOB FOR YOU AT ABUJA
MONSANTO 1,157 reviews – Abuja
Key Responsibilities: Coordinate and execute systems field testing program that supports maize and/or cotton germplasm and trait evaluations across different agroecological zones. Responsible for all aspects of coordination and execution of field trials in alignment with commercial team; trial seed forecasting, location identification, cooperator contracts, trial design, planting, in-season trial maintenance, data recording, harvesting and relinquish data. Active contribution and advancement team’s work, proposals for variety advancements. Maintain country regulatory compliance, ensure trials are conducted within regulatory and stewardship policy and paperwork is accurately completed in a timely manner.
Develop and maintain relationships with Technology Development colleagues to help steward current products and introduce new products. Regularly integrate emerging technology tools to improve work efficiency and execution of assigned responsibilities. Provide technical leadership for local sales organization through agronomic trainings, product and technology scientific knowledge transfer and stewardship initiatives. Technical contribution in demand generating activities like customer visits, demonstration plots, farmer field days and events, marketing tools, etc.
Investigation of product performance inquiries that require specific agronomic and product knowledge. Maintain and build relationship with researchers from academic and research institutions. Ensures equipment is properly maintained, operating and ready for use. Actively participate in the site safety program.
Source=https://ng.indeed.com/job-description? 24 Sept 2017
Aflatoxin in Nigeria=Commodities Certification Points coming
The Federal Ministry of Agriculture has been reported to have the intention of setting up Commodities Certification Centres in each of the 6 geopolitical zones of the country.
This is response to incessant complaints on the unacceptable levels of aflatoxin and pesticides in export agricultural commodities. For some years now The European Union has been frowning at unfair trade coming from Nigeria due to these contaminants. With the putting in place of these standard certification laboratories, it is hoped that compliance with global standards will be attained.
Melon, beans, and a few others have been implicated as notorious candidates for high levels of contaminants from Nigeria to the EU Nigeria
NEW WHO LIST OF RESISTANT BACTERIA THAT NEED URGENT INTERVENTION
WHO Publishes List of Bacteria That Need New Antibiotics
WHO priority pathogens list for R&D of new antibiotics
Priority 1: CRITICAL
Priority 2: HIGH
Priority 3: MEDIUM
The World Health Organization (WHO) has published its first ever list of antibiotic-resistant bacteria that pose the greatest threat to human health and which urgently need new types of antibiotics. These bacteria are resistant to some last-resort antibiotics.
According to the Centers for Disease Control and Prevention (CDC), antibiotic-resistant bacteria kill 23,000 Americans every year. And antibiotic resistant bacteria are present in every country on earth.
The list highlights gram-negative bacteria that are resistant to multiple antibiotics. These pathogens are constantly evolving to resist new drugs, and they can pass genetic material to other bacteria so they can become drug resistant too.
Dr. Marie-Paule Kieny, WHO’s Assistant Director-General for Health Systems and Innovation said in a statement, “This list is a new tool to ensure R&D responds to urgent public health needs. Antibiotic resistance is growing, and we are fast running out of treatment options. If we leave it to market forces alone, the new antibiotics we most urgently need are not going to be developed in time.”
The list is divided into three categories according to the urgency of need to new drugs: Critical, high, and medium priority. The most critical group includes Enterobacteriaceae, which includes Klebsiella and E. coli. Those pathogens can cause severe and often deadly bloodstream infections. Those bacteria have developed resistance to third generation cephalosporins and carbapenems, which are the best available antibiotics for treating multi-drug resistant bacteria.
The second category tier includes Staphylococcus aureus, Helicobacter pylori, fluoroquinolone-resistant Campylobacter, and fluoroquinolone-resistant Salmonella. The third tier includes fluoroquinolone-resistant Shigella bacteria. All of those bacteria are familiar to those in the food safety industry.
WHO hopes that this list will spur governments to advance policies that incentive basic science and advanced R&D by publicly funded agencies such as the FDA and CDC, and by the private sector. Professor Evalina Tacconelli, head of the Division of Infectious Diseases at the University of Tübingen said, “new antibiotics targeting this priority list of pathogens will help to reduce deaths due to resistant infections around the world. Waiting any longer will cause further public health problems and dramatically impact patient care.”
WHO ended its news release by stating that while more research and development is vital, it can’t solve the problem alone. We must prevent infections by these pathogenic bacteria, and use antibiotics in both humans and animals appropriately. And when new antibiotics are developed, they must be used rationally and not overprescribed
Source=https://foodpoisoningbulletin.com/2017/who-publishes-list-of-bacteria-that-need-new-antibiotics/
ELECTRICITY FROM HUMAN WASTES
Dutch designers use human waste to make electricity
By Bongani Shwen
The Compost Kliko Toilet could lead to people getting away from flushing potential down the toilet…
etabolism and cut crop productivity, ultimately putting pressure on arable land…
26 Jun 2017
For more, visit http://www.bizcommunity.com/196/642.html
SEEDS and FERTILIZERS FOR NE FARMERS
FAO distributes seeds and fertilisers to a million people in north-east Nigeria
“Investing in agricultural assistance today will provide food for tomorrow; and can ensure people have a source of food even when they are cut off from other forms of humanitarian aid.”
For more
please visit http://www.bizcommunity.com/Articles.aspx?l=196&c=643&key1=20170703115900
FELLOWSHIP OPPORTUNITY
Food and Agricultural Organisation (FAO) Fellowship Program for Member Countries 2018
Date released=
Food and Agricultural Organisation (FAO) has opened applications for its Fellowship program for typically PhD students, researchers and professors
Application Deadline: 31st December 2017
Eligible Countries: FAO Member countries.
To Be Taken At (Country): Multiple. FAO Regional, Sub-regional, Country Offices or Headquarters.
About the Award: The Food and Agriculture Organization of the United Nations (FAO) leads international efforts to defeat hunger and to support development in member countries in the areas of agriculture, fisheries and forestry. FAO’s mandate is to raise levels of nutrition, improve agricultural productivity, better the lives of rural populations and contribute to the growth of the world economy.
The Fellowship Programme is designed to attract fellows, typically PhD students, researchers and professors, who have an advanced level of relevant technical knowledge and experience in any field of the Organization. They are willing to fulfil their specialized learning objectives and at the same time, contribute their technical expertise and knowledge through time-bound arrangements with FAO. Assignments should be in line with FAO Strategic Objectives and UN Sustainable Development Goals.
Type: Fellowship
Eligibility:
Selection Criteria: Candidates may be assigned in a field relevant to the mission and work of FAO.
Number of Awards: Numerous
Duration of Program: According to time bound agreement with hiring office
How to Apply: To apply, visit the iRecruitment website at http://www.fao.org/employment/irecruitment-access/en/ and complete your online profile.
Visit the Program Webpage for Details
Award Providers: Food and Agricultural Organisation (FAO)
Important Notes:
Aug 20, 2017
Nigeria Mycotoxin Conference holds in November
The Mycotoxin Conference in Nigeria will hold in November, 2017. A reliable source revealed that it will be hosted by the Federal Industrial Research Institute Oshodi FIIRO, Lagos.
Meanwhile the LOC is working on the success, while the theme for the conference will soon be released.
Recall that the last conference was held at Federal University of Technology, Minna, Niger state where a new EXCo was elected
More information shortly
Isolation, screening and identification of Bacillus spp. as direct-fed microbial candidates for aflatoxin B1 biodegradation
Isolation, screening and identification of Bacillus spp. as direct-fed microbial candidates for aflatoxin B1 biodegradation
Isolation, screening and identification of Bacillus spp. As direct-fed microbial candidates for aflatoxin B1 biodegradation (PDF Download Available). Available from: https://www.researchgate.net/publication/282500849_Isolation_screening_and_identification_of_Bacillus_spp_As_direct-fed_microbial_candidates_for_aflatoxin_B1_biodegradation [accessed Aug 9, 2017].
Mycotoxin Control during Grain Processing
Authors=M. E. Tumbleson, Vijay Singh, Kent D. Rausch, David B. Johnston, David F. Kendra, Gavin L. Meerdink, Wanda M. Haschek.
To deal with mycotoxin problems, there must first be an understanding of the fungi which produce them, their growth parameters and interactions with crops. Mycotoxin control is both fungus specific and crop specific. Control of mycotoxins during growing seasons is a crop management problem. Control during storage is…
For more please visit